Course Info
Incomplete
My notes are not a complete account of everything that was taught in this course. Refer to Enderle’s Reader for a comprehensive reading. A few diagrams were borrowed from Enderle’s Reader for convenience during review.
Unit 1: Electrochemistry (Ch. 11)
- electrochemistry
- equivalent mass of metal formed in galvanic cell: molar mass * current * time / Faraday’s constant
11.1
11.2
- find reduction potential using standard hydrogen electrode
- redox reaction
- galvanic cell shorthand
- solve a galvanic cell
11.3
- spontaneity of galvanic cell
- work done by electrons through galvanic cell (happens to equal the change in Gibbs free energy)
- The efficiency of galvanic cell is its max cell potential divided by theoretical max cell ptential.
- Nernst equation (equilibrium constant of standard condition galvanic cell; cell potential of non-standard condition galvanic cell)
- can be calculated using the Nernst equation.
11.4
11.5
11.6
11.7
- electrorefining: “deposition of a pure metal at a cathode from a solution containing the metal ion”
- electroplating: plating one metal onto another (less expensive)
Unit 2: Transition Metals (Ch. 18-19)
18.1 General Properties
- radii: generally follows the periodic trend (with exception)—atomic radii decrease to the right, increase toward the bottom
- lanthanide contraction
- lanthanides have about the same radii as the last row. f-electrons (along with d-electrons) are much less effective at shielding, causing outer electrons to be attracted into the nucleus, and the resultant radii of the third row transition metals are similar to second row metals
- lanthanide contraction
- density: increases then decreases across a d-block row (with exception)
- due to lanthanide contraction, third row has much higher density than second row (around the same volume than 2nd but higher mass)
- oxidation
- transition metals can hold many oxidation states
- second and third row metals are more stable in higher oxidation states
- commong to lose both valence s electrons: oxidation state of +2
- electron configuration
- 4th and 9th column of d-block borrows one electron from s to half-fill or fill d orbital
- 4th column example:
- 9th column example:
- for transition metal ions, remember to remove valence electrons first (e.g. remove from 4s, then 3d)
- shorthand: notation to represent the number of electrons remaining in the d-orbital of a transition metal ion/atom. e.g. has a electron config of and a notation of .
- 4th and 9th column of d-block borrows one electron from s to half-fill or fill d orbital
- magnetism
- Most are paramagnetic (unpaired e-)
- Some are ferromagnetic (has permanent domains)
- catalysis
- TM (transition metals) are very good catalysts
- multiple oxidation states & coordination numbers possible
- ligands can bond easily and have access to multiple bonding sites
- TMs are coordinatively unsaturated (can bond with more ligands)
- TM (transition metals) are very good catalysts
- reactions
- combination
- e.g.
- decomposition (reverse of combination)
- e.g.
- single replacement:
- element reacts with ionic compound / strong acid
- e.g.
- double replacement:
- 2 ionic compounds react to form 2 new ionic compounds. reactants can be strong acids
- e.g.
- combination
18.2 Metallurgy
- metallurgy – refining metal ore to pure metal
- pig iron – result of putting iron ore in blast furnace
- pig iron contains 3-4% carbon
- steel
- formed from pig iron
- carbon content reduced to 0-1.5%
- oxygen is added at high pressure to remove impurities (Si, Mn, P)
- alloys are added (Cr, Ni, Mn, V, Mo, W) to achieve certain properties
18.3 Family Properties
not worth note-taking
19.1 Overview & Terminology (complex ions)
- coordination compound: formed by transition metal (TM) ions + ligands + counter ions; general term for neutral compounds containing transition metals
- e.g.
- complex ions: TM ions + ligands; term used to describe ionic species containing TM
- counter ions: ions needed to produce a compound with no net charge when combined with complex ions
- e.g. the anions from
- ligands: groups (excluding counter ions) that surround transition metal ion
- e.g. the five and one in
- coordination number: the number of neighbors to the transition metal ion; bidentates = 2 and so on; neighbors usually just means ligands in this course
- e.g. in
- complex: general term for species (e.g. ligands) connected to a TM ion
- oxidation state (of coordinate compound): primary valence of transition metal
- has +3 oxidation state in the previous examples
- ligands are usually written with TM ion in square brackets, while counter ions are written after/outside square brackets, e.g. to write the complex as a coordination compound:
- The electron configuration ( shorthand) of the transition metal corresponds to its oxidation number; simply calculate the e- remaining in d-orbital
19.2 Ligands
- TM ion (denoted as M) is a Lewis acid (electron pair acceptor)
- ligand (denoted as L) is a Lewis base (electron pair donator)
- ligands have:
- lone e- pair ready to form a bond
- neutral or negative charge
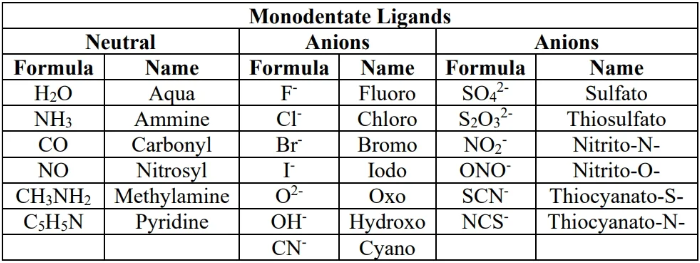
- monodentate: ligand with one lone pair
- bidentate: ligand with 2 lone pairs
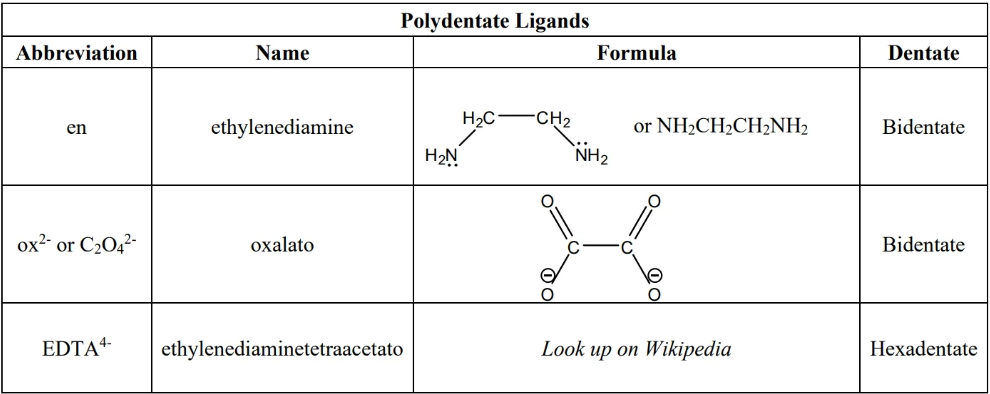
- polydentate: ligand with 2 or more lone pairs
- polydentates are chelating agents
common ligands for this course:
19.3 Nomenclature
- In complex ion and counter ion, name the cations first, then the anions.
- To transform anion names to ligand names:
- -ide to -o
- -ite to -ito
- -ate to -ato
- number of ligands are expressed using a prefix (composite prefix in parentheses)
- mono-
- di- (bis-)
- tri- (tris-)
- tetra- (tetrakis-)
- penta-
- hexa-
- composite ligands—those with prefix(es) in name (e.g. , )—must use composite prefixes. Not all polydentates are composite (e.g. is not composite, so don’t use composite prefix)
- when naming complex ions, name ligands in alphabetical order, and then name metal with oxidation state last
- if complex ion is anion, name the metal with Latin
- iron = ferrate
- copper = cuprate
- tin = stannate
- silver = argentate
- lead = plumbate
- gold = aurate
- if complex ion is cation, name in English with no additional suffix
- if complex ion is anion, name the metal with Latin
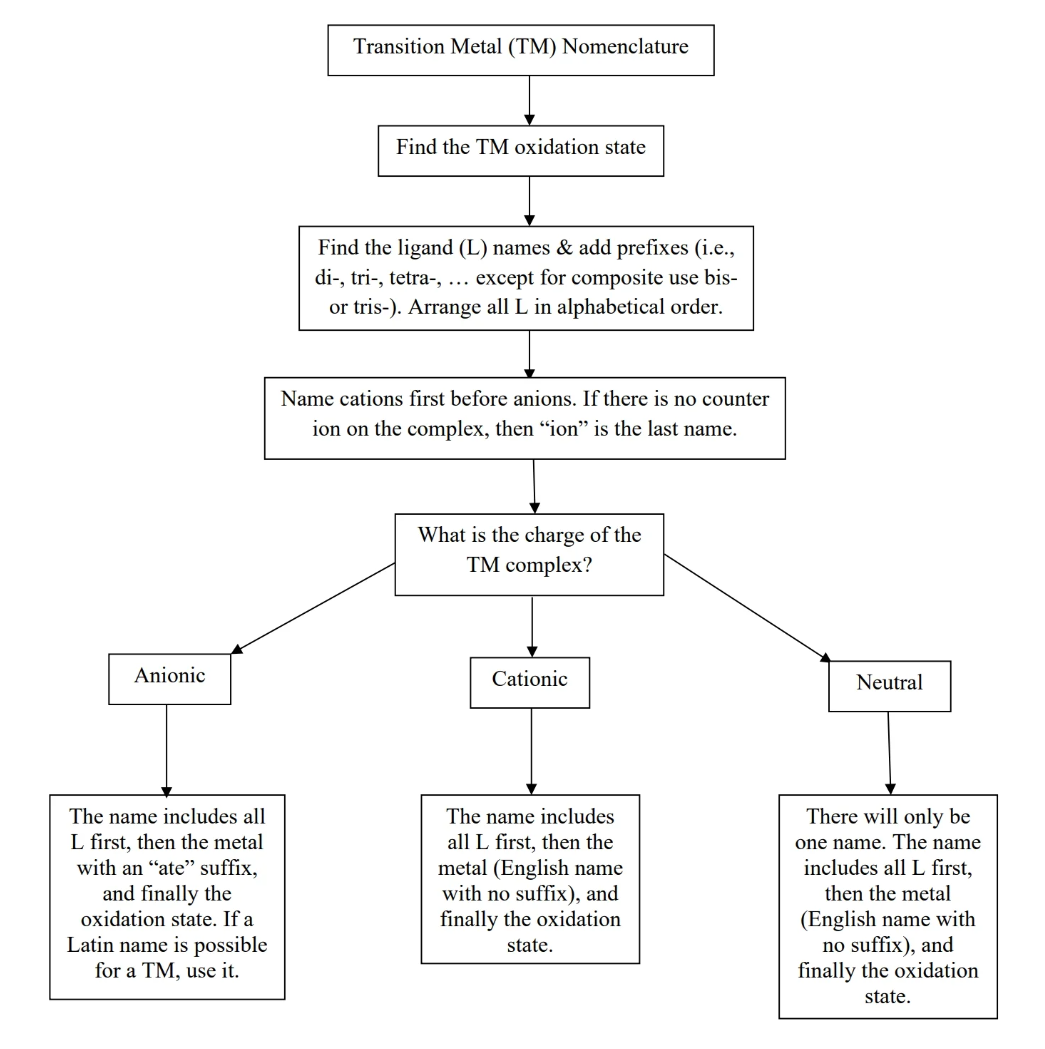

Writing a formula given the name is basically the same thing (cation before anion, transition metal then ligands, etc), except ligands are ordered from most negative, to neutral, to most positive.
19.4 Isomerism
- structural isomers: same chemical composition but bonded differently
- ionization isomers: ligand and counter ion switch
- coordination isomers: a bimetallic (two metal—two complexes) species switch ligands (or view it as the ligands switch the metals); e.g. and
- linkage isomers: a multi-atom ligand connects using different atoms in the ligand, e.g. nitrito-O-ferrate (Fe-ONO) vs nitrito-N (Fe-NO2)
- stereoisomers: same chemical composition, bonded the same way, but different arrangement in space (e.g. chirality)
- geometric isomer: arrangement of ligands are neighboring (cis or fac) or across from each other (trans or mer)
- imagine the transition metal of the complex at the origin of a coordinate system
- cis/trans isomerism: only occurs for square planar & octahedral complexes where ligands can be on the same plane
- cis: ligands are bonded neighboring each other (e.g. one is 90 degrees from another)
- trans: ligands are bonded across from each other (lies on the same axis)
- fac/mer isomerism: octahedral complexes, considering 3 ligands
- fac: 3 identical ligands bonded to the metal neighboring each other (i.e. bonds not on the same axis)
- mer isomerism: 3 identical ligands bonded to the metal, where the bonds are on the same plane (2 on the same axis, one is not); think “meridian”
- chirality is only considered for cis and fac (where ligands are neighboring)
- optical isomers: a.k.a. enantiomers, can be called chiral/optically active; the mirror image of the molecule cannot be superimposed onto the original; in other words, mirror images of the molecule produces a differently arranged isomer
- a tetrahedral is chiral if all four ligands are different
- an octahedral is chiral if any is true:
- all 6 ligands are different
- 2-3 same monodentate ligand neighbors
- only 2 monodentate ligands are the same and they are cis (neighbors)
- only 3 monodentate ligands are the same and they are fac (neighbors)
- 3 (any 3, same or different?) cis pairs
- 3 bidentate ligands
- 2 bidentate ligands (cis position)
- 1 bidentate ligand plus different trans monodentate ligands plus different cis monodentate ligands
- isomers may be simultaneously geometric and optical
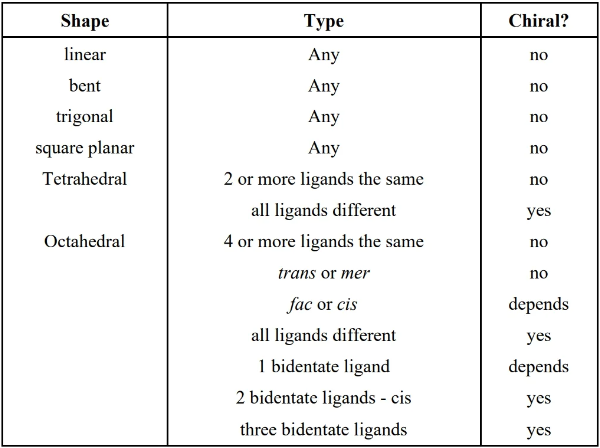
- geometric isomer: arrangement of ligands are neighboring (cis or fac) or across from each other (trans or mer)
19.5 Crystal Field Theory
- 5 d orbitals:
- on the axis (degenerate): x^2-y^2, z
- off the axis (degenerate): xy, yz, xz
- in crystal field theory, there are different configurations for each VSEPR shape
- is the difference between the highest and the lowest energy, the energy range of energy levels so to speak
- if is smaller than pairing energy (weak field), then electrons prefer to go to higher energy levels—electrons don’t double fill orbitals until all d orbitals are single-filled since pairing cost more energy than going to higher energy levels
- if is larger than pairing energy (strong field), then electrons prefer to pair: double fill lower energy orbitals first before filling higher energy orbitals
- we don’t know by default
- when the ligand is known, we can determine relative amount of by looking at the spectrochemical series, which arranges ligand species in order of . and above is considered high . Low is below (water and below)
- for metals, increases down a group
- for oxidation state, increases as oxidation state increases
- octahedral: 6 ligands and 1 metal
- each ligand bond sit on the axis (positive/negative)
- since ligands are trying to bond on the axes, off-the-axis orbitals have lower energy (minimal overlap)
- tetrahedral
- ligand bonds are not on the axis (due to weird bond angles)
- since ligands are trying to bond off the axes, on-the-axis orbitals have lower energy (minimal overlap)
- square planar on xy plane
- metal in middle, each ligand bond sit on either or axis
- highest energy: x^2-y^2 (directly on x and y axes)
- next highest energy: xy (on the xy plane)
- next highest energy: z^2 (the large poles are on z but the negative region donut overlaps with x-y plane)
- lowest energy: xz, yz (no direct overlap with xy plane)
- linear on z-axis
- both ligands on z axis
- highest: z^2 (majority of orbital on z axis)
- next highest: xz, yz (close to z axis)
- lowest: xy, x^2-y^2 (nothing to do with z)
19.6 Magnetism
- paramagnetic: has unpaired electrons
- diamagnetic: all paired electrons
- strong field = diagmagnetic (more paired electrons)
- weak field = paramagnetic (fewer paired electrons)
- the more unpaired electrons, the more paramagnetic
19.7 Color
We can use from CFT (spectrochemical series of the ligand) to calculate the wavelength of photon that TM complexes absorb (and thus determine the color of the metal).
If increases, decreases.
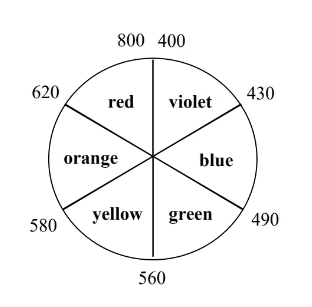
- In daily life, seeing a certain color means that the substance is absorbing the opposite color on the color wheel. For instance, seeing yellow means that the substance took away violet light from sunlight (white light, which contains colors of all spectrum).
- If a metal complex absorbs UV light, then it will appear white because no visible spectrum light is absorbed from ambient illumination.
- An increase in (e.g. switching from a weak field ligand to a strong field ligand) causes to decreases, which results in a bluer color.
- When calculating , make sure is the absorbed color and not the reflected color.
Unit 3a: Main Group Metals
Section 1 - Alkali Metals
H, Li, K, Na, Rb, Cs, Fr
flame colors
- With an appropriate input of energy (), s-electron of alkali metal gets excited to p-level. When electron returns to original level, it emits a photon. This property is used to make fireworks colorful.
bonding
- Since there is one valence electron, there is weak bonding within the metal, which leads to some properties listed below.
- Low densities (floats on water)
- Soft
- Low melting points
oxidation
- easily oxidized (only one e-), low ionization energy
- (oxidation potential) is positve & large which makes oxidation spontaneous.
- +1 oxidation state
Formation of oxides
- readily form oxides depending of radius of metal atom
- list of oxide ions:
- oxide ion:
- peroxide ion:
- superoxide ion:
- major combustion products for alkali metals
- (oxide)
- (peroxide)
- (superoxide)
- (superoxide)
- (superoxide)
reactions
- Na is produced through decomposition of
- K is produced through single replacement from Na
application (soap & detergent)
- Soap & detergents are produced through reaction of fat and NaOH (a.k.a. lye).
- Lye is used to dissolve fat and make soap (also used to take care of roadkills, and even corpses by some criminals)
- product is a soap molecule with two parts: a long organic chain and a carboxylic anion with a sodium cation
- example:
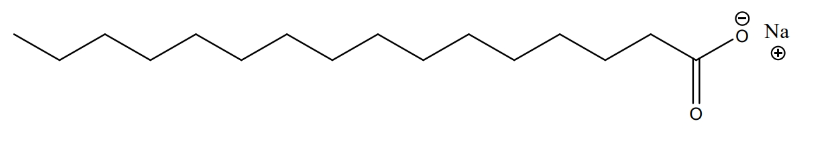
- ionic part is polar and dissolves in water
- organic part is nonpolar and dissolves in dirt and oil (also nonpolar)
- the molecule is useful for cleaning since it has both a polar & nonpolar part (can bind with both types of substances)
Hydrogen
- not much similarity with any group
- nonmetal
- colorless, odorless, tasteless
- flamable
- industrial production of hydrogen
- (water gas shift reaction)
- liquid at 20K (low London forces, hard to stay together)
- light (1/10 density of water)
- liquid H used as fuel in space, together with liquid O2. combustion between the two propels the spacecraft
Section 2 - Alkaline Earth Metals
Be, Mg, Ca, Sr, Ba, Ra
properties
- +2 oxidation state
- low density
- low melting point
- low boiling point
- insoluble substance
- does not decompose upon heating
- generally produced by reduction
- exception: Beryllium
- higher melting point
- harder
- unreactive towards air & water
- smaller radius
- high ionization energy
- compounds tends to be more covalent than ionic
- limited tendency to form
- has a unique structure: 2 Cl atoms are on a plane perpendicular to the rest
- exception: radium
- radioactive
- properties not well established
applications
- make concrete with CaO + sand + gravel + water
- chalk / limestone: calcium carbonate
- decomposes into quicklime (with CO2 byproduct) at high temperature, used for metallurgy
- lime reactions
- formation of quicklime, used in water treatment
- formation of slaked lime (calcium hydroxide) (cheapest commercial strong base) from quicklime;
- Rainwater (acidic) protonates strong calcium carbonate in ground to form calcium bicarbonate. Dissolution of calcium carbonate forms underground caves, stalactites (on ceiling), and stalagmites (on floor).
- barium sulfate is opaque to X-rays. Low concentration solution (so that it stays insoluble in human body) is useful for X-raying internal organs.
- formation of quicklime, used in water treatment
Section 3 - Hard water
- hard water: water containing alkaline earth metals
- hard water causes issues in household
- build up on water containers:
- from calcium carbonate and magnesium carbonate causes build up (e.g. in pots and pans after being used to boil water).
- To reduce/remove buildup, rinse/heat pan with vinegar (acidic) to neutralize the carbonate (basic).
- Buildup may also occur in pipes, which restricts water flow and causes corrosion.
- soap doesn’t work as efficiently when used with hard water
- soap + magnesium ions & calcium ions = precipitates
- common deposit locations: bathtubs (body wash, etc), clothes/fabric (detergent)
- soap bubbles/foam is also harder to form
- the harder the soap, the more soap is needed
- some detergents are made to stay functional in hard water
- build up on water containers:
- ion exchange column: porous material filled with NaCl (need to be replenished)
- part of household hard water removal solution (water softener)
- NaCl dissociates into ions, Na+ adheres to the column
- Hard water ions displaces sodium ions, so sodium ions takes the place of unwanted ions in the water (sodium doens’t cause any problems, except for plants). Water that exits the column becomes soft water.
Main Group Reactions
- general combination reaction (where is any metal)
- common combination reaction forms
- simple (no byproducts)
- ( anion)
- metal + water
- metal compound + water
- simple (no byproducts)
- decomposition
- general example:
- reaction rules & tips
- most importantly, product side should have an ionic compound with:
- metal cation in its common oxidation state
- anion in its common oxidation state
- water + metal = metal hydroxide, sometimes with additional product (, , ) without which the equation could not be balanced
- non-metal species will join the metal on product side
- different alkaline metals form different oxides (oxide, peroxide, superoxide)
- make sure that the equation is balanced (check subscripts in products)
- most importantly, product side should have an ionic compound with:
Section 4 - Group 13 Icosagens
Al, Ga, In, Tl
- common oxidation states: +1, +3
- +1 is more common due to inert pair effect: post-transition metals tend to not share the valence s electrons
- relatively soft
- good conductors
- relatively reactive
- compounds made of icosagens can form dimers (esp. Al), where two identical compounds bond together
aluminum
- most abundant metal in crust
- strong but light
- forms alumina ()
- formed by electrolysis
- reducing agent
- found in precious gems: when (partial, e.g. ~1%) replacement of alumnium occurs in corundum (), a new gem is formed
- ruby: replacement with ()
- sapphire: replacement with
- topaz: replacement with
gallium
- obtained by smelting
- higher density in liquid phase (like water)
- can weaken other metals by infiltrating & diffusing into their crystal lattice
- has various applications in electronics
indium
- squeals when bent
- oxidation state determines whether it is a reducing or oxidizing agent (strong at both)
- has various applications in electronics
thallium
- greek word for “twig”
- highly toxic (was once used as poison)
- has various applications in electronics
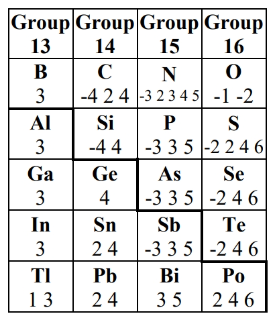
Inert Pair Effect
- Post-transition metals & semimetals (groups 13-16) tend to not involve s-subshell valence electrons in reactions (e.g. sharing, redox). This means that most of the time, only the p-electrons are used in reactions for these elements.
- We must take the inert pair effect into effect when determining the oxidation state
- Post-transition metals can have up to two different common oxidation states (the smaller one doesn’t involve s-electrons, and the bigger one does)
- Metalloids can have negative and positive oxidation states.
Section 5 - Group 14
Sn, Pb
- formed by roasting then reduction
- common oxidation states: 2, 4 (by inert pair effect)
Tin (Sn)
- application: food storage, solder, pewter (Sn alloy)
Lead (Pb)
- applications: batteries, ammunitions, pipes
- poisoning: lead may interfere heme in blood and cause nervousness, depression, and even nerve damage or kidney failure
Unit 3b: Main Group Nonmetals
Section 1 - Group 18 (noble gases)
He, Ne, Ar, KKr, Xe, Rn
group properties
- odorless
- colorless
- monatomic
- relatively unreactive (full octet) but not completely inert. in order for compounds to form:
- need an ionizable noble gas atom (the larger the atom, the better; Xe is best, Rn is radioactive so can’t form stable compounds)
- need an atom with high electronegativity (O or F)
- examples: , , ,
- low mp
- low bp
- low intermolecular forces
- nearly ideal gas at STP
rest skipped (element info)
Section 2 - Group 17 (Halogens)
F, Cl, Br, I, At
- halogens are denoted by letter X
- produced industrially by electrolysis or oxidation
- is most reactive (since it’s the least polarizable), while iodine is the least reactive
- HF is useful for glass () etching.
- in halogen compounds, halogens are ordered from least to most electronegative element (e.g. , )
- halogens + oxygen = oxy acids
oxy acid pattern (replace “hal” with the corresponding halogen name, e.g. HOF = hypofluorous acid)
| oxy acid | ox. state | name |
|---|---|---|
| HOX | +1 | Hypohalous acid |
| HOXO | +3 | Halous acid |
| HOXO2 | +5 | Halic acid |
| HOXO3 | +7 | Perhalic acid |
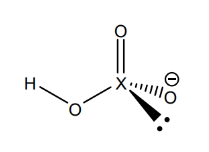
example oxy acid lewis structure (perhalic illustrated)
Section 3 - Group 16 (chalcogens)
O, S, Se, Te, Po
oxygen
- ozone protects us from UV ray energy
- oxygen oxidation states: 0 (O2), -1 (, i.e. peroxides), 2 (most common)
sulfur
- most common form is S8, though Sn where n = 1, 2, 6, 2000-5000 also exist.
- sulfur oxides: there are many, but common oxides are sulfur dioxide and sulfur trioxide
- sulfur oxoanions: there are many, but common ones are sulfite (), sulfate (), and thiosulfate ()
- emissions of and lead to acid rain
- once used in chemical warfare
selenium & tellurium are used in semiconductor, alloys, glass colorings
polonium is a radioactive chemical
Section 4 - Group 15 (Pnictogens)
N, P, As, Sb, Bi
- can have multiple oxidation states
nitrogen
- either extremely stable (e.g. N2) or extremely reactive (e.g. explosives like nitroglycerin, TNT; reactions are fast, exothermic, with high volume expansions and stable products)
phosphorus
- common oxidation states: +3, +5 (inert pair effect)
skipped
Section 5 - Group 14 (Tetragens)
C, Si, Ge
carbon
- essential to life
- forms strong bonds
- forms cyclic and acyclic structures
- can bond with itself and other atoms
- forms single and multiple bonds
- allotropes: graphite, diamonds, nanotubes, graphene, Buckminsterfullerenes
- highest sublimation point of all elements
silicon
- there are many silicon oxides (silicates) formed through different combinations of tetrahedra
- zeolites: aluminosilicates (, )
germanium
- similar chemical properties to Sn, Si
Section 6 - Group 13 (Icosagens)
B
boron
- common oxidation state: +3, though it behaves more like silicon than aluminum
- electron deficient
- can have incomplete octet
- can be classified as metalloid or nonmetal
- can form dimers and covalent allotropes
- covalent bonding (nonmetal characteristic)
Unit 4: Kinetics
- (instantaneous rate of apperance/disappearance of one chemical is the derivative of concentration w.r.t. time divided by its coefficient in the reaction equation; remember to set the sign to negative for if it’s a reaction side formula unit/disappearance)
- average rate = secant line =
- rate laws
- for a reaction , the rate , where and is the order of the reactants in the reaction.
- is the rate constant, whose units depending on the overall order of the reaction (e.g. add )
- Rate constant depends on the specific reaction, the current temperature, and whether or not catalysts are present
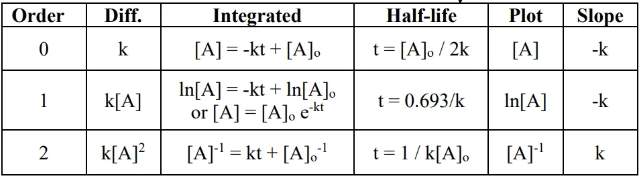
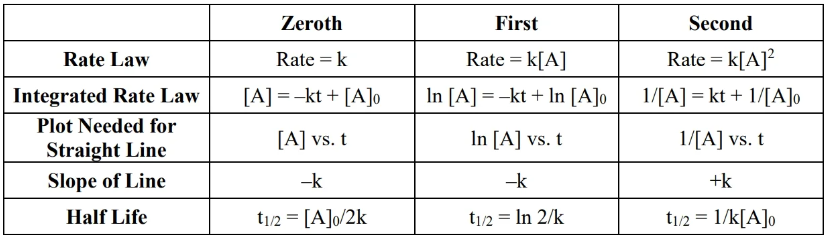
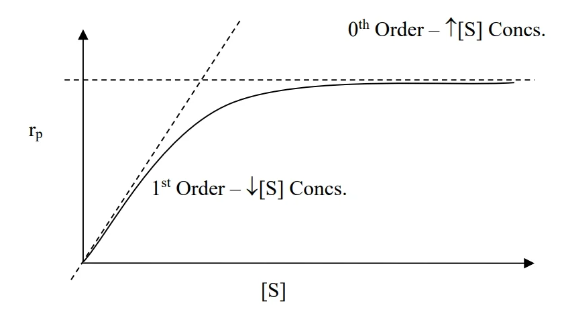
- To determine overall order of reaction, make three plots (refer to table at top) vs time and see which has the right slope (refer to slope column).
- zeroth order rate law () — fixed rate
- for a reaction ,
- reaction rate does not depend on reaction concentration
- example: metabolism of alcohol in body, which only happens at a fixed rate
- unit:
- to verify that a reaction is zeroth order: plot vs and check that the slope is
- differential rate law
- rate law that describes how the rate changes with respect to one reactant’s concentrate
- The larger the (and therefore ) are, the faster the reaction goes.
- determining order of reaction from table of reactant concentrations vs rate of appearance of product (M/s)
- The easiest way is to find two rows (trials) where only one reactant’s concentration changes, write the differential rate law, plug the reactant’s concentration & overall reaction rates in, and divide the two trials’ rate law equations. Factors related to columns/reactants that didn’t change in concentration will be cancelled since they stayed constant in both trials. Solve for the order .
- Sometimes there is no pair of trials such that only one column change. In this case, find pairs such that minimal number of columns change (make sure that different columns change for different trials), and divide them. Solve the system equation for
- reaction mechanisms
- rate of appearance of one side = rate of disappearance of another side
- rate-determining step:
- rate-determining step is the slowest reaction step
collision theory
- given a reaction
- A and B must collide in the right orientation
- A and B must collide with enough energy
- factors of sucessful reaction
- collision frequency: the more frequently the molecules collide, the more chance the molecules has at starting a reaction
- activation energy
- threshold kinetic energy increases with temperature
- Boltzmann distribution:
- as temperature increases, average velocity increases, and more molecules (right tail of std distribution) will pass the KE threshold.
- threshold kinetic energy increases with temperature
- orientation: the correct atoms must impact at the right orientation
transition state theory
- In transition state theory, when A and B first collide, a transition state complex (only partial bond) a.k.a. an activated complex forms. At the transition state, the reaction could succeed (form products) or fail (return to reactants)
- transition state is denoted by double dagger symbol ()
- doesn’t work
- quantum mechanics: reaction could succeed if transition state theory requirements are not fulfilled
- at higher temperature, there are so many possible energy states that the expected reaction path may actually be leading to a different product
reaction profile
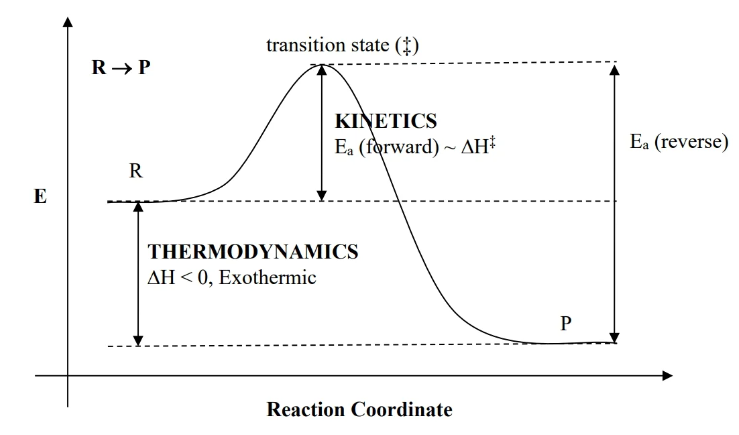
- enthalpy is difference between R and P. If P is lower than R, it’s exothermic, vv.
- : activation energy (which is also )
- transition state is a peak of activation
temperature and reaction rate
- Arrhenius equation
- k increases with T (temperature)
- is the frequency factor that depends on
- : frequency of overall molecular collisions
- : frequency of collisions with proper orientation
- the exp term is the fraction of sufficiently energetic collisions
- activation energy
- R is the ideal gas constant
- Clausius-Clapeyron equation
- reaction mechanism
- a reaciton mechanism describes how reaction is done and consists of a series of elementary steps/elementary processes/reaction steps
- elementary process requirements
- consistent with overall reaction
- account for rate law
- elementary process properties
- uni- or bi-molecular
- has the same coefficients of formula units as the rate law (i.e. order of reaction comes from coefficients of reaction)
- reversible
- intermediates may appear (species that do not appear as a reactant or product in the overall equation)
- slowest (small r/small k) reaction step = rate-determining step (RDS)
- solve for RDS
- deduce the slowest step
- write rate law for the slowest step
- if any, substitute away the intermediates
- if reversible reaction is present, set forward & reverse rate law equal to each other to solve for an expression for intermediate. note that reverse reaction should have a different rate constant (e.g. becomes )
- use PSSH to solve for rate law of a step (not necessarily RDS / doesn’t need to be RDS)
- pseudo steady state hypothesis (PSSH) / steady state assumption frees us from making assumptions about RDS. PSSH states that at equilibrium, intermediates’ rate of appearance (of elementary step products) and rate of disappearance (of element step reactants) are equal
- e.g. if forward reaction of step 1 produces a intermediate that can be consumed by step 2, then
- if we know that , we know that most intermediates produced by forward reaction are not available for the second step (since they are consumed in the reverse reaction), so the slow step/RDS is the second one, and the step’s rate law can be simplified (remove terms with a coefficient from all linear combinations)
- for more difficult scenarios, try combining the two techniques above
catalysis
- catalysts are speeds up reaction by letting the reactants take a different and less costly path to form products
- catalysts are used in a step then formed in another, so no net consumption
- homogeneous catalysis: reaction and catalyst are in same phase (e.g. liquid and liquid)
- heterogeneous catalysis: reaction and catalyst are in different phases
enzyme catalysis
- enzymes
- like catalysts, but biological
- positions the substrates in the right positions to make a reacion occur quickly (?)
- the opposite: inhibitors decrease rate of reaction by binding to enzymes
- enzymes and inhibitors do not need to be removed from rate laws
- Michaelis-Menten kinetics: model for enzyme kinetics
- E: enzyme, S: substrate, P: product
- typical reaction involving enzyme and substrate
- (step 1)
- (step 2)
- rate law for product:
- RDS: , we need to remove intermediate
- use PSSH
- is typically not known, but (initial amount of enzyme) is
- solve for
- , so
- plug that back into the equation of rate laws
- plug back into rate law
- substitute in
- when substrate concentrations are low,
- resulting reaction is first order (varies only the substrate concentration)
- when substrate concentrations are high,
- zeroth order (constant rate of production)
- where enzyme is most efficient
Unit 5: Intro to Organic Chemistry
Oh no.
molecular spectroscopy: a method of determining molecular structure
| molecular spectropy type | radiation |
|---|---|
| nuclear magnetic resonance | radio |
| molecular rotation | microwave |
| molecular vibration | infrared |
| electronic transitions | ultraviolet |
| electron spin resonance | microwave |
OChem common molecular spectropy:
-
NMR: nuclear magnetic resonance
- Nucleus has a nuclear moment (spin). The correct radio wave will align and energize spin.
- determines C-H framework of molecule
-
IR: infrared spectroscopy
- we can measure vibrational states under infrared radiation
- determines functional groups in molecule
-
UV/Vis: UV Spectroscopy
- electrons in conjugated systems are excited to higher energy states
- determines if a conjugated system is present in the molecule
-
conformation/conformers: identical molecules that are rotated differently in space
-
isomers: compounds that are different but have the same formula; different arrangement or connectivity in space
- structural isomers are differently bonded have different properties
- geometric isomers: different isomers with groups in either cis or trans configurations
carbon classification
- carbon can be classified by the number of carbons bonded to it
- from least carbon to most: primary (), secondary (), tertiary (…), quaternary
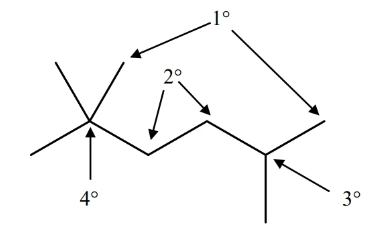
name alkanes by the number of carbons in chain
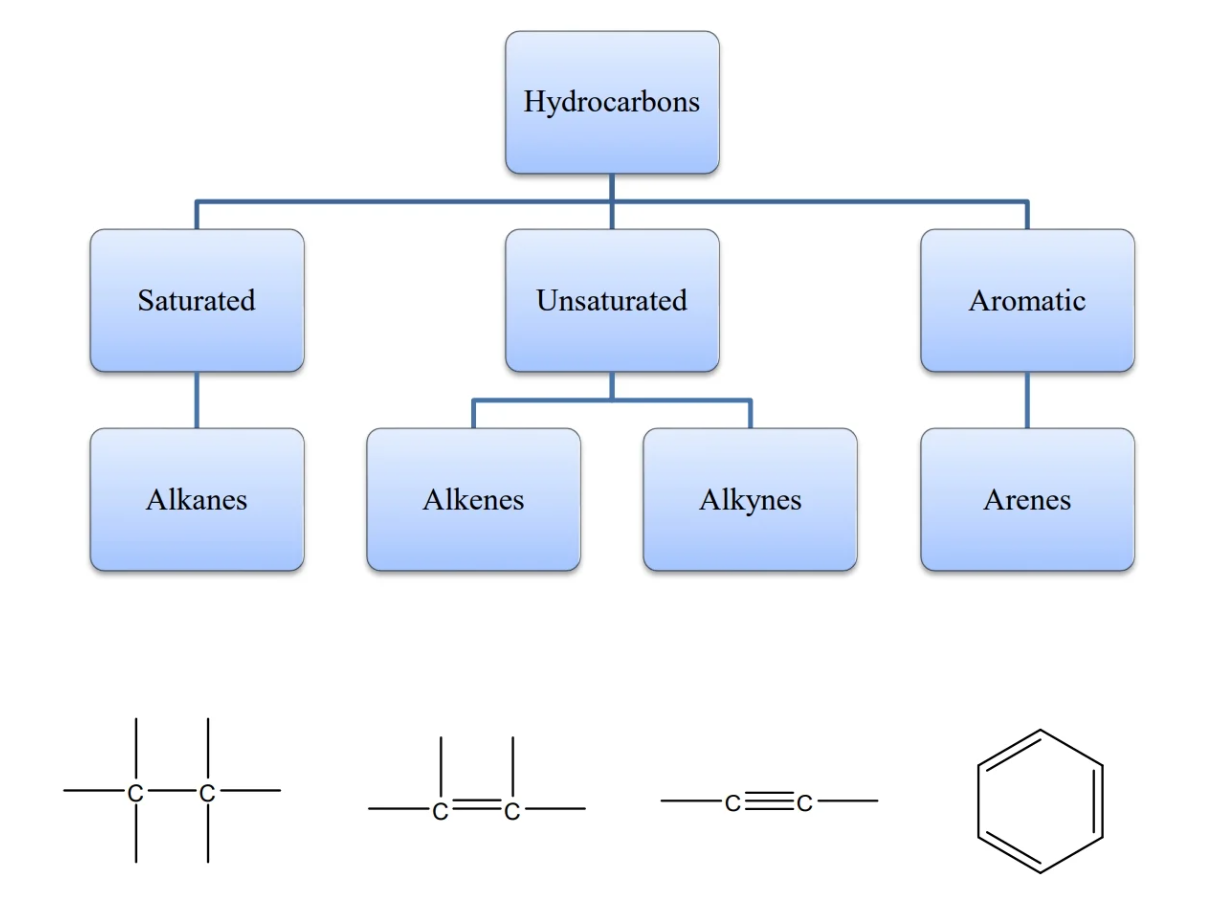
- alkanes: family of molecular structures that only contain single-bonded carbons and hydrogens
- all alkanes have the suffix -ane
- relationship between H to C in alkanes: H = 2C + 2
- Each C in the middle has two H bonded up and down. Each C (there are two) at the end of the chain need an additional hydrogen to satisfy the octet.
- alkyl (denoted R) are groups that are alkanes
special alkyl groups:
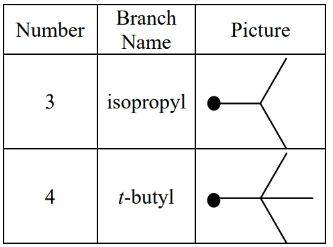 in -butyl (a.k.a. tert-butyl) is not counted when sorting alphabetically (it’s considered a prefix), but the i in isopropyl is
in -butyl (a.k.a. tert-butyl) is not counted when sorting alphabetically (it’s considered a prefix), but the i in isopropyl is
alkyl prefixes
Alkane IUPAC nomenclature
- name longest chain
- name all substituents (i.e. branches)
- determine carbons’ numeric positions (used to name substituent positions) based on these precedences; this set should have the lowest sum of substituent positions compared to other numerc position sets
- find the longest & most substituted chain to set the numeric positions
- carbon with a functional group closest to an end of chain should have the lowest numerical position possible (i.e. start numbering close to functional group, don’t start on the other side)
- if no functional group exists or if functional groups are on symmetrical positions (ambiguous), the carbon bonded other substituents (e.g. alkyls) closest to an end of chain should also have the lowest numerical position possible ater functional groups
- lastly, if ambiguity still remains, set position numbers so that the substituents are named in alphabetical order
- name the molecule in this order
- name substituents from lowest position to highest
- format for each substituent
- numerical positions of substituent molecules on the longest & most-substituted chain
- comma-separated
- the prefix (e.g. the tri- in triethyl) + substituent
- numerical positions of substituent molecules on the longest & most-substituted chain
- format: positions-substituent- … positions-substituent
substituentincludes the prefix- name position-substituent pairs in alphabetical order sorted by substituent name (not including the prefix)
- no hyphen necessary between name of last substituent and the name of chain
- omit position if there is only one substituent and the substituent is at the first position (does it only apply for cycloalkanes?)
- format for each substituent
- the name of the longest chain
- name substituents from lowest position to highest
naming example:
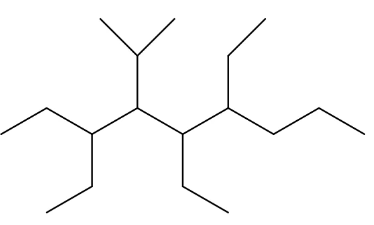 3,5,6-triethyl-4-isopropylnonane
3,5,6-triethyl-4-isopropylnonane
cycloalkane:
- alkanes with a cycle
- add prefix to alkane name: cyclo-
- starts with cyclopropane (at least three vertices/carbons needed for cycle)
cycloalkane IUPAC nomenclature
- pick and name parent ring
- name substituents
- Number the carbons on ring so that carbons bonded to functional or alkyl groups have lowest possible number. Note that a carbon with multiple substituents should have a lower number than carbon with one or no substituents.
- check that this is indeed the lowest set by compare sums of substituent positions
- name the cycloalkane with the same template as regular alkane
general properties of organic molecules
- organic molecules can combust with oxygen (exothermic)
- organic molecules dissolve each (all relatively unpolar; like dissolves like)
properties of alkanes & cycloalkanes
- alkanes have the formula . As increases, size, London force, and mp & bp also increase.
- cycloalkanes
- have the formula .
- experiences bond strain
- e.g. C in cyclopropane is sp3 hybridized, but has bond angles of 60 degrees when the ideal should have been 109.5 degrees. This means that bonds tend to buckle (bend out of shape) to relieve the strain.
- Since alkanes and cycloalkanes stack well with themselves, they have higher mp than branched (substituted) alkanes.
alkene properties
- alkene: hydrocarbon that contains C=C double bonds
- more reactive than alkanes since alkanes don’t have a functional group
- has a double bond which means no free rotation around that bond
alcohol
- solubility is high in water (i.e. miscible in water) when the OH group dominates (i.e. there are fewer than 4 carbons)
polymer properties
- alkenes form many well known polymers
halogenation, a.k.a. free radical halogenation
- a reaction between alkanes and halogens
- or reacts with alkane in presence of light () to form organic halides
- the halogen replaces an hydrogen on the alkane
- halogen prefers to take place of hydrogen bonded to the carbon that has the most carbon neighbors (i.e. tertiary > secondary > primary; quarternary is not possible since there wouldn’t be any hydrogen in the first place)
addition reaction
- alkene reacts with molecule that has two parts e.g.
- the reagent molecule gets split up into A and B, then the two parts are added to each side of a double bond (carbon)
- the C=C double bond becomes a single bond
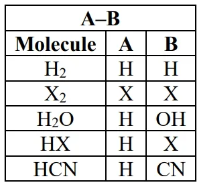
substitution reactions
-
(specifically ) occur when nucleophile (Nu; reagent with lone pair, sometimes negative) substitutes for a leaving group (L; group that can be easily lost by the organic molecule).
-
-
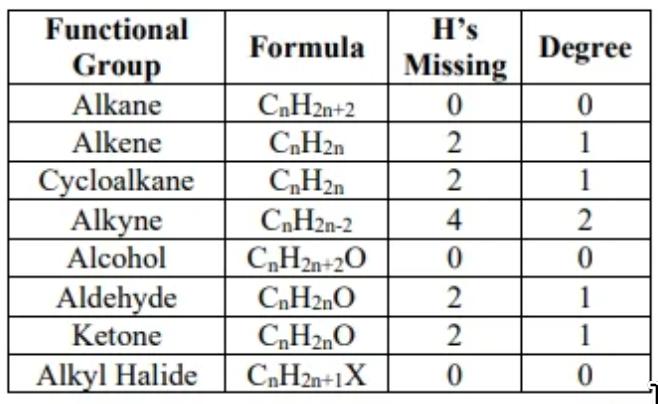
degree of unsaturation:
- An alkane is of the form . If an organic molecule has fewer hydrogen than an alkane with the same carbon count, then the molecule is not an alkane, and the number of missing hydrogens is indicative of whether or not double or triple bonds exist. An alkane is considered fully saturated (i.e. with hydrogens, halogens, groups, etc).
- degree = (number of hydrogens in alkanes - number of hydrogens in species) / 2
- halogens are considered hydrogens in unsaturation calculation
chirality
- molecule has a enantiomer if its mirror image cannot be superimposed on another
- if carbon (the stereocenter) is bonded to 4 different groups, then the molecule is chiral
- chirality designation/naming:
- R = right/rectus/name clockwise
- S = left/sinister/counterclockwise
- check if a molecule is R or S
- Rank atoms connected the stereocenter by molar mass from highest (#1) to lowest (#4) atomic number. If any atoms are the same, rank them by the atoms bonded to the former.
- if #4 is not pointed away from you (i.e. dashed line bond), redraw so that it is (e.g. by rotating the diagram).
- Trace from #1 to #3. If the trace is clockwise, then it’s R, L otherwise. If any redrawing was done, then the trace is actually opposite (if got R, then it’s actually S).
Unit 6: Nuclear Chemistry
quantum tunneling causes emission: nucleons bypassing/teleporting across the normally forbidden regions (i.e. outside nucleus)
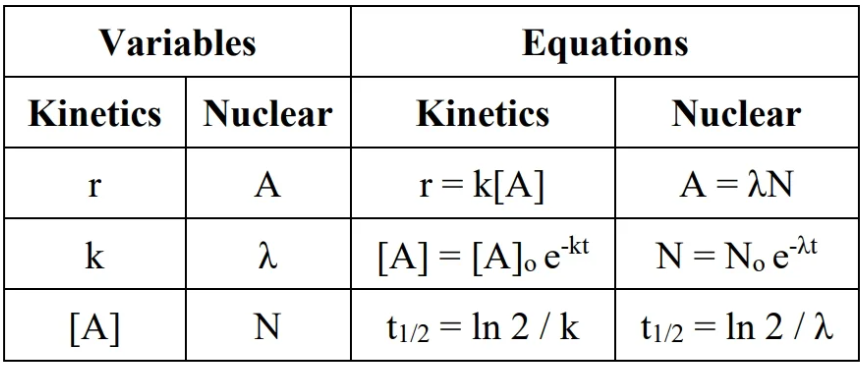
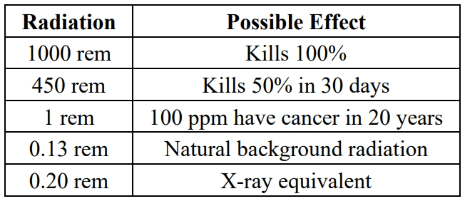
units for radiation:
radioactive dating
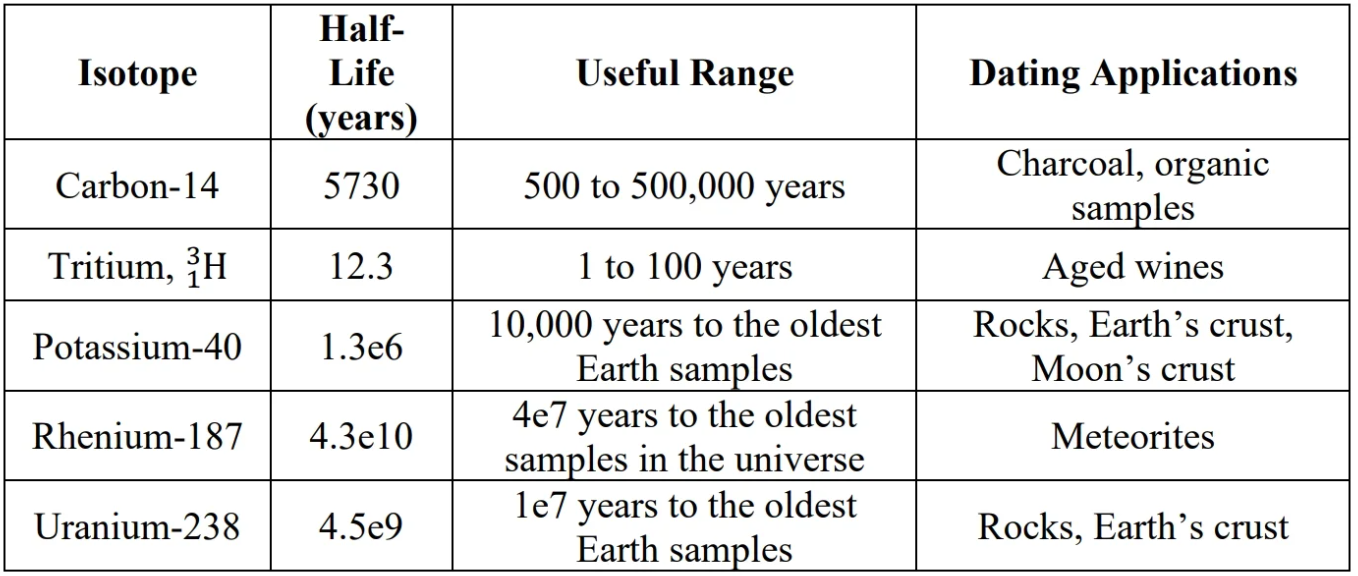
Carbon-14 dating:
- Neutrons from cosmic radiation causes Carbon-14 to form.
- decay:
U-238 dating:
U-238 decays to stable lead isotrope when the molten it’s in solidifies. Ratio of Pb-206/U-238 determines the age of the rock.
To find how much energy is emitted by the lost mass:
- find the difference between product and reactant mass
- either use or convert the mass to the appropriate unit
- : use (where is the atomic mass unit)
- : use
- : divide J/nuclei result by number of nucleons (mass number)
nuclear binding energy: energy needed to combine nucleons and form a nucleus general problem format:
Note that negative sign is often unnecessary since the energy is understood as energy lost.
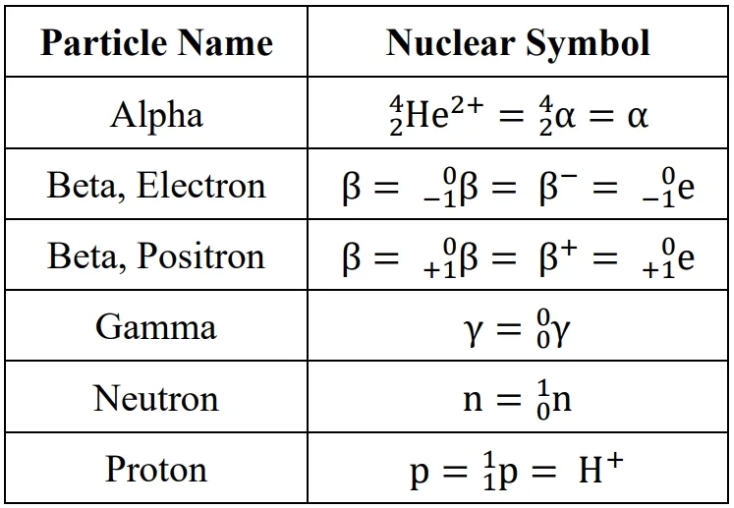
particle types:
unnamed heavy (transuranium) element naming:
(based on atomic number digits):
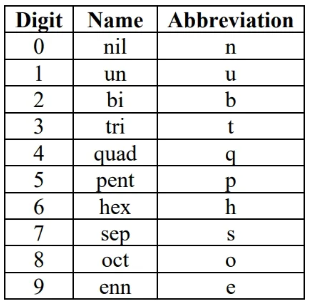 symbol is the concatenation of digit abbreviations
symbol is the concatenation of digit abbreviations
nuclear stability
- If Z > 83: definitely radioactive
- If A and Z matches periodic table: likely stable
- If Z = N and Z ⇐ 10 (or 20): likely stable
- radioactive exceptions: and
- there are many stable (non-radioactive) exceptions where Z != N
- If Z or N equal a magic number: likely stable
- double magic even more stable
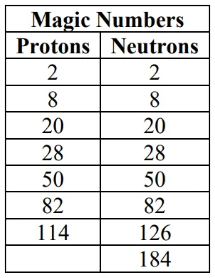
- If Z and N are even: may be stable
- If either of Z and N are odd: may be radioactive
- If Z and N are both odd: likely radioactive
effect of radiation (rem = radiation equivalent to man)